More Information
Submitted: 26 November 2019 | Approved: 04 December 2019 | Published: 05 December 2019
How to cite this article: Ejiugwo M, Shaw G, Barry F, Krawczyk J, McInerney V. The motivational factors and adverse events experienced by healthy volunteers donating bone marrow for research. Int J Bone Marrow Res. 2019; 2: 089-096.
DOI: 10.29328/journal.ijbmr.1001010
ORCiD: orcid.org/0000-0003-4586-4091
Copyright License: © 2019 Ejiugwo M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Bone marrow donation; Mesenchymal stromal cell research; Motivational factors for bone marrow donation; Healthy volunteers; Adverse events
Abbreviations: BM: Bone Marrow; CRFG: Clinical Research Facility Galway; HSC: Haematopoietic Stem Cell; IC: Informed Consent; LBP: Low Blood Pressure; MSC: Mesenchymal Stromal Cell; NMDP: National Marrow Donor Program; PBSC: Peripheral Blood Stem Cell; SD: Standard Deviation; WMDA: World Marrow Donor Association
The motivational factors and adverse events experienced by healthy volunteers donating bone marrow for research
Mirella Ejiugwo1, Georgina Shaw2, Frank Barry2, Janusz Krawczyk3,4 and Veronica McInerney4-6*
1Centre for Research in Medical Devices (CURAM), National University of Ireland Galway, Ireland
2Regenerative Medicine Institute, National University of Ireland Galway, Ireland
3University Hospital Galway, Ireland
4School of Medicine, National University of Ireland Galway, Ireland
5HRB Clinical Research Facility, National University of Ireland Galway, Ireland
6School of Nursing and Midwifery, National University of Ireland Galway, Ireland
*Address for Correspondence: Veronica McInerney, HRB Clinical Research Facility, School of Nursing and Midwifery, National University of Ireland Galway, Ireland, Tel: + 353 86 803 1890; Email: [email protected]; [email protected]
Background: With the advancement of cell therapy research, there is an increasing need for healthy volunteers (HV) to donate small volumes (30 ml) of human bone marrow (BM). The BM procedure required to procure small volumes is invasive, although short-lived (25 seconds), is not without risk. To ensure a sustainable supply of BM for research and cell therapy, greater information of the risks and factors that motivate HV to donate small volumes of BM will help optimize the procedure and HV enrolment, ensuring donors are fully informed of the potential risks.
Objective: To identify the adverse events (AE) experienced by HV during and after small volume BM procedure and understand the motivating factors that influence HV to donate BM for research.
Method: HV (n = 55) who donated BM (30 ml) for scientific research and provided informed consent were administered a questionnaire to identify the type, duration and severity of AE experienced during and post-BM aspiration; and to determine the motivating factors that influenced their willingness to donate BM.
Results: Pain was experienced by 89% of participants during the BM procedure with moderate grade reported by 40%. One/more of the following AE were experienced by 73% of the volunteers post-BM procedure: pain, fatigue, site reaction, nausea and transient hypotension. AE resolved within an average of three days. The reported motivational factors ranked in the following order: first, to advance research for the benefit of future patients; compensation for participation; free medical check-up; lastly, the research question was interesting.
Conclusion: Young HV, motivated primarily by altruism and financial compensation, risk the occurrence of transient AE following donation of small-volume BM for research.
Stem cell-based therapies are a rapidly growing category of complex cellular medicines used to treat a wide variety of diseases: peripheral vascular, autoimmune, neoplastic, cardiovascular, pulmonary, ophthalmological, hepatic and neurological diseases, and haematological disorders - to name a few [1,2]. Diverse tissue sources of stem cells exist, such as fat tissue, bone marrow (BM), perinatal tissues, foreskin, dental tissue, salivary glands, etc.
In particular, the human BM, a spongy tissue rich in key cells of the immune system and site of red blood generation found in bones, has historically been donated by healthy bone marrow donors for haematopoietic stem cell (HSC) transplantation – a treatment approach for some haematological cancers and other illnesses. Advances in cell-based therapies have led to the extraction of therapeutically promising, mesenchymal stromal cells (MSCs) from the human BM. Given their availability, ease of expansion, amenability to genetic/tissue engineering, and regenerative potential, MSC have been widely used since 1995 in a variety of clinical trials in the treatment of diseases [3,4]. In advancing the clinical potential of MSCs, researchers require healthy BM tissue for development of new cell based regenerative therapies and understanding of the mechanisms and safety of these therapeutic regimens. The development and manufacturing of MSC-based allogenic therapies is also dependent upon the availability of healthy BM tissue for use as the ‘starting material’.
The volume of marrow required for biomedical research and allogenic therapies is substantially less than that required for HSC transplants. The procedure requires skilled personnel. Using local anaesthetic, a small volume of marrow (30 mls) is procured from just one puncture wound in the healthy donor’s iliac crest. Although the procedure is short-lived, lasting approximately 25 seconds, it is an invasive procedure and carries with it the risk of adverse event occurrence to the healthy donor.
Heretofore, data to support the risks of bone marrow procurement from healthy donors has been informed primarily by the experiences of individuals undergoing large volume donation for BM harvesting for HSC transplantation. This is a well-established procedure and considered generally safe and well-tolerated [5,6]. BM donors are generally screened first for relevant serological conditions such as hepatitis and cytomegalovirus and, more recently, for sickle cell anaemia [7]. The donor may be related or unrelated to the recipient and under general anaesthetic, large volumes of marrow up to 20 - 27 ml per kg of the donor’s weight is aspirated by a series of multiple punctures from the bilateral posterior iliac crests of the donor’s hip bone using large bore BM needles [8]. According to the World Marrow Donor Association (WMDA) guidelines, the recommended duration of anaesthesia for such a procedure should be less than 150 minutes, with the aspiration procedure not lasting more than 120 minutes [9]. Although described as ‘safe’, procedural complications associated with this type of large volume marrow donation are categorised as major and include: infection, anaesthesia, transfusion, mechanical injury, and sacroiliac joint and sciatic nerve, chipped teeth, urinary retention [9]. These complications are supported by the work of other researchers [10]. Other events such as fracture of the ilium post-aspiration, mechanical injuries to the bone, malignant hyperthermia, cardiopulmonary problems, bacterial infection and cerebrovascular events have also been reported as relatively rare but life-threatening complications [6]. It is estimated that the risk of death with BM donation for this purpose is approximately 1 in 10000. Until 2005, six deaths were reported following cardiac arrest, ventricular fibrillation, respiratory arrest, myocardial infarction and pulmonary embolism in large volume BM donors [11].
Less severe but common complications reported with large volume bone marrow donation include: excessive pain at the marrow aspiration site, transient low blood pressure, fatigue, severe post-spinal headache, fever, fainting, unexpected hospitalization, minor infections, and difficulty/pain while walking or sitting - which resolved within few days of onset [12,13]. Lisenko, et al. [14], purport that high quantities of BM aspiration lead to anaemia-related side effects such as fatigue, hypoxia, exhaustion and dizziness [14]. Individuals who donate large volumes may be hospitalised between 1 and 19 days [6,14,15]. The volume procured is positively associated with pain, hypovolemia, fatigue, pain while climbing stairs, difficulty walking, insomnia, and fainting days following the procedure [15]. Less positive psychosocial reactions of donors is associated with longer periods of collection [16]. Multiple punctures of the iliac crest and anaesthesia are associated with pain, restricted activity and longer periods of hospitalisation [17,18]. The longevity of symptoms vary in reports with some donors returning to their baseline health-related quality-of-life status in less than three months [19], while others report BM donors continued to experienced pain at the collection site 6 months after the donation [20]. Age and gender are also associated with the level of experienced toxicities, experienced: women were more likely to feel tired while older donors had lingering pain seven days after the procedure [21].
While ample data is evident on adverse events experienced by donors of marrow for transplant purposes, information on the type and rate of adverse events experienced by healthy individuals undergoing small volume bone marrow donation for research purposes is not at all well documented. This information is necessary, not only to optimize the donation procedure but also to ensure the healthy volunteer is fully informed of the potential risks he/she will be exposed to.
Motivations for bone marrow donation
Similar to the data surrounding adverse events, the motivations for donating BM for HSC transplant use is very well researched [21,22]. Individuals with personal connections with patients who need blood-based products and individuals with less family responsibilities are more likely to donate BM for HSC transplant use [23]. Differences have been found between males and female donors. Better self-image and lower levels of pre-donation ambivalence were found in donors who reported empathic motives and positive perception before the donation. It was also seen that more educated donors and younger donors reported fewer positive reactions 12 months post-donation [22]. Females donate for altruistic and normative considerations, experience positive individual feeling following the humanistic act and have empathetic feelings for the recipient or the recipient’s family [22]. Female however are also less willing to donate to an unrelated recipient and experience more pain and physical restrictions than males [19]. Males feel like better people after donating marrow, have lower levels of pre-donation ambivalence, more positive donation experiences and report less physical difficulty with the procedure [22]. Garcia, et al., reported that volunteers donate marrow primarily as a social obligation such as saving a person’s life, providing a good act of kindness and reinvesting their good health in others. It can be seen a means of expressing charity and benevolence towards others and repaying God for one’s life. Donors related to recipients donate out of a sense of family loyalty. Their perceived responsibility toward their loved one outweighed all risks of the donation procedure. Some donors used this as a technique to build positive self-identity and to validate personal ideals, self-confidence and ego [24].
Motivations thus far relate to large volumes of BM required for transplant use. No data could be found for those donors who donated smaller volumes of marrow for research purposes and/or for the expansion of stem cells for clinical trials.
Maintaining an active pool of eligible BM donors to meet the biomedical research and clinical manufacturing demand for BM-derived MSCs is necessary for the sustainability of research and development in cell therapy. As such it was proposed to conduct this study to identify risks that healthy volunteers may be exposed to and adapt procedures if necessary to minimize those risks. This study also aims to determine and rate factors that motivates young healthy volunteers to donate small volumes of marrow.
Study design
This was a retrospective study of healthy volunteers who donated small volumes (up to 30 mls) of bone marrow for the purpose of research at the HRB Clinical Research Facility Galway (CRFG) between 2011 and 2019.
Study objectives
The primary objectives of the study was to identify motivating factors that influence healthy volunteers decision to donate BM for research and to determine adverse effects related to the bone marrow procurement.
Population and setting
From a database of one hundred and sixty healthy volunteers who had donated BM for research between the years 2011 and 2019 at the CRFG, sixty were contactable by telephone and invited to partake in the study. Fifty-five (n = 55) consented to take part in this telephone-based study.
Questionnaire design
The primary objectives of this study was to identify the adverse events, rate, duration and severity, experienced by health volunteers during and after donating small volumes of BM for research and to determine the motivational factors for donating marrow. Demographics captured included gender, age, nationality and employment status of volunteers at the time of their BM donation was procured.
Adverse events experienced during and after the marrow procedure were captured using a researcher developed questionnaire which was based on the published data of events reported by healthy volunteers who donated large volumes BM for HSC transplant. These events were: pain at the marrow collection site, site reaction, skin rash, infection, fainting/loss of consciousness, fatigue, bleeding, hypotension, nausea and vomiting [12-18,20]. Research participants who experienced pain following the procedure were asked to rate the severity of it on a Likert scale of 1 to 10. Questions relating to the duration of experienced side effects, restriction in activity and hospitalization were also included.
A Likert scale (1 to 10) was used to assess research participants’ understanding of both short-term and long-term risks of the BM donation procedure as explained by the researcher prior to undergoing it.
To assess what motivated participants to donate small volumes of BM for biomedical research of MSC, a validated questionnaire by Soule, et al. [25], was used (with the author’s permission). With this questionnaire, participants rated their motivation for donating BM on the Likert scale of 1 to 10 (1 signifies “totally disagree” and 10 “strongly agree”) to each of the following items:
“I signed up because it was an interesting study and research question.” (Q1)
“I signed up because of the compensation for participation.” (Q2)
“I signed up because findings from the research might be able to help future patients.” (Q3)
“I signed up because it would be helpful to have free medical check-up.” (Q4)
Nomination of other motivations by participants was sought using an open-ended question. The questionnaire also interrogated research participants’ willingness to donate again, whether they would recommend marrow donation to their family and friends and how they found out bone marrow was needed for research.
Ethical submission and approval
The study protocol along with letter of invitation and participant questionnaire was submitted to Galway University Hospital Research Ethics Committee for approval. Following receipt of positive opinion and approval by ethics committee, study enrolment commenced.
Data protection and confidentiality
Participant details and questionnaire responses were treated confidentially in line with data protection legislation.
Data and statistical analysis
The frequency of reported adverse events following the BM donation and the mean recovery time were calculated.
Firstly, relevance scores were calculated to assess how important each motivation for volunteering was, compared to the other motivations. The relevance scores were calculated by subtracting the item score of a motivation (e.g. Q1) from the average score of the remaining three motivations (Q2, Q3 & Q4): relevance score of Q1 = Q1-[(Q2+Q3+Q4)/3]). A negative relevance score for a motivation signified that a research participant was more inclined with the remaining motivations than it. Conversely, a positive relevance score means that the research participant was strongly influenced by the concerned motivation compared to other motivations.
To rank the motivations in order of priority for volunteering, firstly the individual relevance scores for each motivation were ranked as 1st, 2nd, 3rd and 4th for each research participant. The total number of times each motivation was ranked 1st, 2nd, 3rd and 4th in all participants were counted. Then, the number of times that each of the four motivations were ranked either the 1st or 2nd positions were summed; the same was repeated for the number of times each of the four motivations ranked either 3rd or 4th positions. The obtained two counts per motivation were compared and the higher of two was chosen. The selected counts of the four motivations were then ordered: ranging from the greatest value meaning the corresponding motivation that was most strongly the reason for volunteering (1st position) to the least value, which signified that the corresponding motivation was the weakest determinant for which healthy volunteers took part in the study (4th position).
The association between gender and each of the four motivations for donating BM for MSC research was assessed using 2-sample t tests. Resulting p values that were less than 0.05 were considered statistically significant. All data and statistical analyses were performed on Minitab (Minitab Inc, State College, PA, 16801, USA) version 17.0.
Research participants characteristics
Out of the population of interest, successful contact was made with sixty individuals; fifty-five (n = 55) provided informed consent and agreed to take part in the survey. Participants’ demographics are presented on table 1.
| Table 1: Demographics of the 55 research participants. Healthy Volunteers Who Donate Small Volumes of Bone Marrow for Research and Clinical Trials Use. |
||
| Demographics | % (n) | % (n) |
| Gender | Male 43.6 (24) | Female 56.4 (31) |
| Profession | Student 80 (44) | Worker 20 (11) |
| Country of Origin | European 91 (50) | Non-European 9 (5) |
| Second-time BM donation | Yes 16.4 (9) | No 83.6 (46) |
There was no significant difference between genders. 80% (n = 44) of participants were students at the time of their BM donation and 20% of participants were working in paid employment. Age ranged between 18 and 25 for 82% (n = 45) of participants with the remaining participants (18%) aged between 26 and 35. Donors were 80% Irish, 11% Polish and 9% non-European. Nine of the 55 healthy volunteers had donated their BM twice.
Motivating factors for bone marrow donation
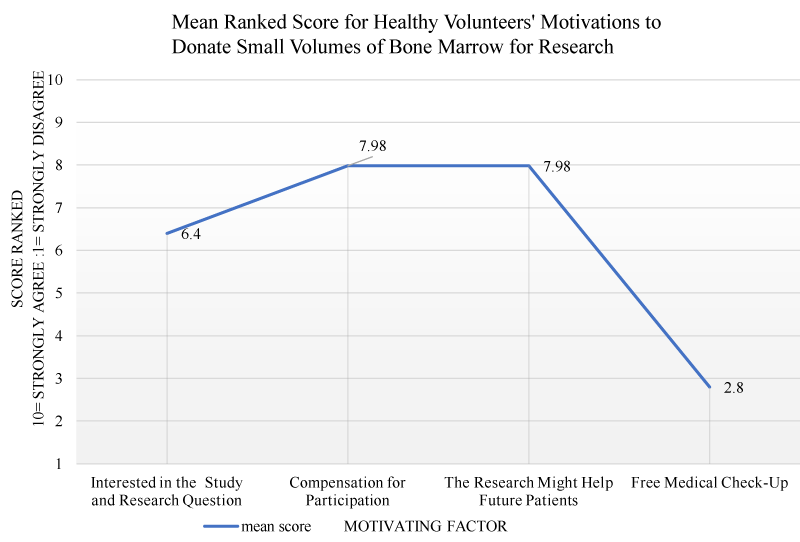
Participants ranked the factors that motivated them to donate bone marrow for research on a scale of 1-10. The higher number indicated stronger motivation. Some participants gave equal ranking to more than one motivating factor. The mean rankings for each motivation is presented on figure 1.
Figure 1: Coomassie brilliant blue stained SDS-PAGE gel.
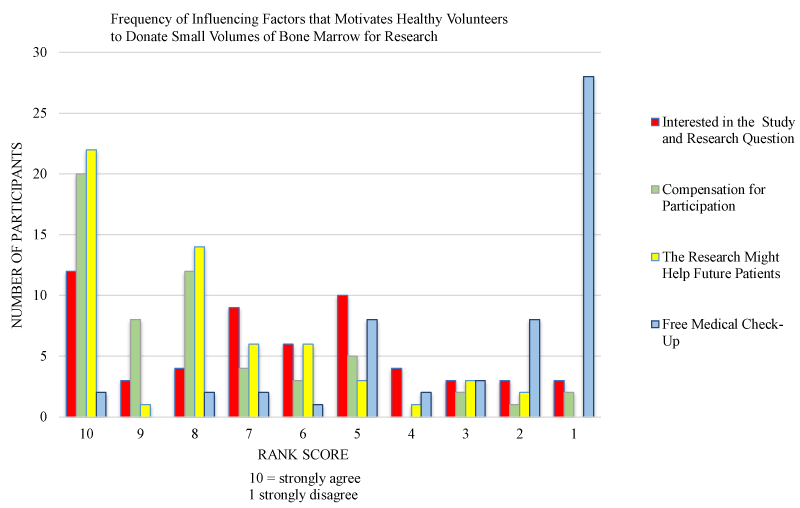
The order of motivations are presented in table 2. Based on the available data, altruism ‘the findings from the research might help future patients’ was ranked as the highest ranking motivating factor amongst participants with 98.2% (n = 54) following the combination of times it ranked both 1st and 2nd positions. This was followed very closely by financial motivation with 87.3% (n = 48) of participants strongly agreeing they donated BM for financial compensation. ‘Free medical check-up’ ranked as the third motivating factor for BM donation by 70.9% (n = 39) of participants. Donating BM because ‘it was an interesting research question’ was the fourth and weakest, and ranked with 61.8% (n = 34) strongly agreeing this was a motivating factor. There was no significant difference between males and females for any of the motivational factors. The frequency of infleuncing factors that motivates healthy volunteers to donate small volumes of bone marrow for research were grouped according to ranking and presented in figure 2.
| Table 2: Factors that motivate healthy volunteers donate small volumes of BM for research and clinical trial use. Combined ranking for first and second positions, and the third and fourth positions for the four motivations from the relevance score data are presented. The highlighted values indicate the relatively higher ones that are arranged orderly to identify the order of motivations for BM donation for research. |
||
| Motivation | 1st and 2nd Position Ranking Combined | 3rd and 4th Position Ranking Combined |
| Future benefit to science (Q3) | 98.2% (54) | 1.8% (1) |
| Compensation for participation (Q2) | 87.3% (48) | 12.7% (7) |
| Interesting study (Q1) | 61.8% (34) | 38.2% (21) |
| Free medical check-up (Q4) | 29.1% (16) | 70.9% (39) |
| 1st position: To advance scientific knowledge for the future benefit of patients; 2nd position: Compensation for research; 3rd position: Free medical check-up; 4th position: Interesting research question. | ||
Figure 2: Factors motivating health volunteers to donate BM for research. The bars represent the scores given by research participants under the ranking score categories of 1-4, 5-7, and 8-10 – regarding the assessed four motivations for donating their BM for research. 10 = strongly agree; 1 = totally disagree.
Other additional motivational factors were nominated by participants: three participants were motivated by the need for volunteer to the bone marrow project (which could be come under the remit of altruism). One participant wanted to experience the procedure in case future donation is needed for any of his family members. One donor who was working the oncology ward, wanted to undergo the experience of his patients (empathy). One participant wanted to know his pain threshold. While another participant volunteered because his friends already had donated BM donation, therefore, he was curious to experience the same (social obligation).
Events experienced during the bone marrow harvesting procedure
Participants were asked to categorise the severity of pain experienced during the BM aspiration procedure. Severity was categorised as ‘no pain’, ‘minimal/mild pain’, ‘moderate pain’, and ‘severe/disabling pain’. 39.5% participants reported they experienced moderate pain during the procedure. The findings are presented on table 3.
| Table 3: The events experienced by the 55 healthy donors during the bone marrow aspiration procedure. Events experienced during the Bone marrow procedure. | |||
| Level of pain | % (n) Male |
% (n) Female |
% (n) Combined |
| No pain | 5.4 (3) | 3.6 (2) | 9.1 (5) |
| Minimal/mild pain | 10.9 (6) | 20 (11) | 30.9 (17) |
| Moderate pain | 14.5 (8) | 25 (14) | 39.5 (22) |
| Severe/disabling pain | 10.9 (6) | 7 (4) | 17.9 (10) |
| Cannot remember pain felt | - | 1.8 (1) | 1.8 (1) |
Events experienced after the bone marrow procedure
27% (n = 15) of participants reported that they did not experience any adverse events or complication related to the BM aspiration procedure while 73% (n = 40) reported a single or combination of events post-donation. The adverse events reported were pain, fatigue, site reaction, nausea, and transient low blood pressure (LBP), numbness, stiffening of the hip joint, difficulty in walking. The frequency of these events are presented on table 4. No bleeding, infection, vomiting, skin rash or fainting was reported.
| Table 4: Frequencies of side effects in the 40 healthy volunteers that experienced any symptom after the BM harvesting procedure. Adverse Events Experienced by Healthy Volunteers After Donating Small Volumes (30 mls) of Bone Marrow. |
|
| Participants experienced adverse events post marrow donation | “Yes” 73% (n = 40) “No” 27% (n = 15) |
| Event | % (n) |
| Pain | 67 (37) |
| Fatigue | 14.5 (8) |
| Site reaction | 18 (9) |
| Nausea | 3 (2) |
| Low blood pressure | 1 (1) |
One female participant reported the need to attend physiotherapy due to significant pain and inability to bend after the procedure. Amongst the nine second-time BM donors, one experienced hip stiffness and another slight difficulty in walking after the second donation.
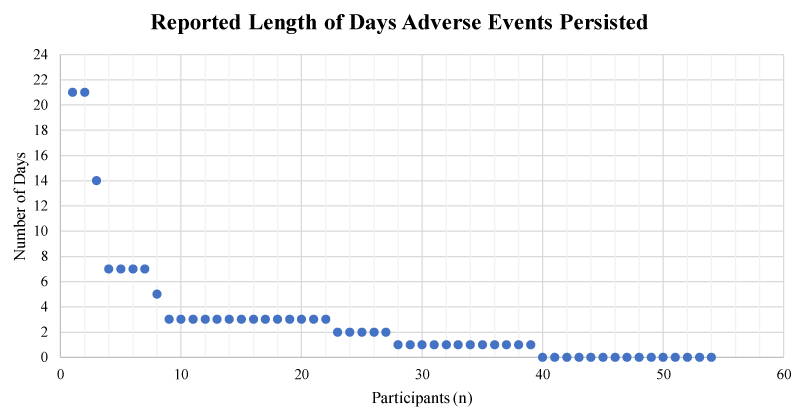
Pain felt primarily in the hipbone near the site of bone marrow procurement was the event of highest prevalence. The mean pain score rating out of a scale between 1 and 10 was 3.8 (SD = 1.56). 18% (n = 10) of participants also reported some restrictions in mobility due to these side effects with the median length of restriction being 1 day. The mean time period reported for complete resolution of side effects was approximately 3.8 days post-procedure; however, 2 participants reported pain lasted up to 3 weeks (Figure 3).
Figure 3: The reported number of days that experienced adverse events persisted in BM donors (n = 55).
There was no significant difference in the association of gender and adverse events. 89% (n = 49) heard about need for BM donors by word of mouth and volunteered for BM donation. Participants strongly agreed that they had in-depth understanding of both short and long-term risks of the BM aspiration procedure with a mean score of 8.8 (SD = 1.5) out of 10. Despite the reported side effects, 78.8% (n = 45) expressed willingness to donate their BM again, if opportune, and 91.2% (n = 52) would recommend donating BM to their family and friends.
BM-derived MSCs have been identified as an attractive investigational product for use in clinical trials for diverse disorders [26]. The growing demand of MSCs will necessitate a stable supply of BM as a starting material. Availability of BM is dependent upon people’s willingness to donate it. This study demonstrates the motivating factors that contribute to healthy volunteers donating BM in order of priority these are: 1) to advance research that could benefit patients in the future (altruistic motivation); 2) compensation for research (financial motivation); 3) to receive free medical check-up (health-related motivation); 4) the research question was found interesting (intellectual motivation).
In congruence with the selfless concern for the well-being of others and the desire to improve the quality of life of others reported by BM donors for HSC transplantation [22,24], altruism was the leading motivating factor for donating BM reported in this study. It has been suggested that participants may morph their motivations to sound altruistic to either suit the socially desirable behaviour or meet the expectations of the clinical researcher [27]. The researcher in this study had no previous interaction with, and was not known to participants. In addition to this, interviews were by telephone which further minimized personal interaction, thereby negating the need for participants to provide anything other than a true response.
The second leading motivational factor for donating BM was financial compensation. This finding is supported by that of other studies where healthy volunteers were majorly motivated to participate in research for financial benefit [28]. It has been suggested that fair compensation for enduring the uncomfortable procedure and contributing to the common good sounds reasonable [29] and apart from the prospect of financial compensation, participants of research most likely consider other factors such as risks, potential health benefits and inconvenience [30]. As evidenced in this study, BM aspiration was reported as a painful surgical procedure and participants reported an in-depth understanding (mean score of 8.8 ± 1.5 out of 10) of the short-term and long-term risks before providing their informed consent. It could be purported that upon weighing the risks, payment may have influenced the decision to donate BM. The WMDA opposes remuneration of BM donors for HSC transplantation, amongst other reasons, to avoid the impairment of public’s will to act altruistically and to prevent coercion and exploitation of donors [31]. The BM donated by volunteers in this study was not for HSC transplantation but rather it was for research and science. Given the context, the pain and effort volunteers are exposed to, grounds the belief that compensation is justifiable.
Participants in this study were less than 35 years of age and primarily students. However, the eligibility criteria for the BM project were healthy volunteers between 18 and 35 years of age, and the advertisement was circulated within the local University (NUI Galway). Therefore these demographics should not be mistaken as a greater likelihood that younger volunteers or students to donate BM. It could be suggested that financial compensation is a motivational factor with this demographic.
The motivational factor that ranked third was free medical check-up. Similar to other BM donation studies, free check-up consists of a thorough means of the screening process for volunteers prior to BM procurement procedure - i.e. serology testing for infectious markers, full blood count, biochemistry check, blood pressure and vital signs check, and physical examination by the physician [11]. The participants who gave higher scores for this motivation were prevalently medical students – in the present research study, this observation is justified by the prevalent student population that donated BM for research. This study took place in Ireland where social health care system is in place. This motivation factor may be stronger in countries where medical insurance is mandated.
Intellectual motivation (i.e. “I signed up because I found the research question and study interesting”) ranked fourth and was deemed the least relevant motivational factor for donating BM for basic research. A clear understanding or curiosity of the reasons for procuring BM (e.g. treating certain diseases with human MSC from human BM, etc.) should support the idea of helping to advance research in favour of future patients. As such, it would be expected to find intellectual motivation closely linked to altruism, in which case this should have been the second most motivating factor for BM donation. The observed schism between the two motivations in the present study is worth-investigating further.
Adverse events
Retrospectively, most participants reported experiencing moderate pain during the BM aspiration procedure. Moderate pain levels have been statistically associated with individuals with mononuclear cell count greater than 2.7 × 109/L [9]. This was not explored in this study. Contrary to findings of previous studies [9], severe/disabling pain was reported by more male donors than their female counterparts during the BM harvesting procedure. Despite of that, most donors agreed to donate BM again. This may be indicative of the positive psychosocial effects of their previous marrow donation, tolerability of the procedure and minimal burden of side effects experienced. The remaining participants who stated that they would not donate again reported either feeling moderate or severe pain during the BM aspiration procedure or had longer recovery times post-donation.
Common symptoms reported by donors of large volume of BM under general anaesthesia for HSC transplantation include back pain, bleeding and pain at collection site, fainting, nausea, headache, fever, light-headedness, bandage pain, vomiting, pain sitting, infection, intravenous site pain, anaemia and sore throat [11]. Typically, the complete recovery time of BM donors for HSC transplantation has been between two and eight weeks; on rare occasions, up to twenty four weeks [9]. In this study, BM volunteers had a maximum of thirty mls of marrow procured under local anaesthetic and the events reported were pain, fatigue, site reaction (redness or swelling around the BM collection site), nausea and a single case of transient hypotension. The average period reported by volunteers to return to their normal activities was three days, a significantly shorter time period than that required for HSC donation. No event of bleeding, infection, vomiting, skin rash, fainting or hospitalization was reported. The absence of infection, signifies that aseptic technique, sterile materials and environment were used for the aspiration of BM at the research facility.
It has been suggested that pain post-donation could be intrinsically connected to a BM donor’s mental preparedness before the procedure [24] and that donors report less pain related distress if they are provided with a description of the pain in addition to procedure [32,33]. In this study, low levels of pain was the most frequently reported adverse event post donation.
As supported in previous studies [5,9,11,34], fatigue, nausea and site reaction experienced by participants in this study can be attributed to the local anaesthesia used during the BM harvest procedure. In summary, the side effects following the procedure of collecting BM for research can be described less severe compared to those reported by BM donors for HSC transplantation. This can be attributed primarily to the difference in the number of BM collection sites and the volume of BM collected and number of aspirations. It is important to add, that no volunteers required hospitalisation post procedure, all were discharged home, and there was no medical follow up required for any of them.
A larger sample size study would strengthen the generalizability of findings [35]. The sampling period was for participants who donated marrow between 2011 and 2019. Although the survey approach was cost-effective and efficient, this time interval may give rise to recall bias as it has been suggested that memory of the side effects may disappear 12 months post-donation [16]. Administering the questionnaire to the healthy volunteers at the time of the marrow donation and within on- month post donation is recommended for future studies and would minimise this risk. This would also eliminate, unrealized challenges of general database protection regulations (GDPR) for contacting participants retrospectively.
In this retrospective study of fifty-five participants, forty-nine participants heard about BM donation by word of mouth. This is perceived as an indicator that participants found the procedure acceptable and without long term health risk. A prospective study to explore motivational factors more in depth including a comparison of compensation and recruitment rates of healthy volunteers for BM procurement internationally would be valuable and could contribute toward developing best practices for volunteers in research.
The motivational factors that contribute to healthy volunteers donating BM in order of ranking are: (1) Altruism, to advance research for the benefit of future patients; (2) financial compensation for participating; (3) Intellectual motivation- the research question was of interest to the volunteers and; (4) Free medical check-up. Reported adverse events following BM donation for MSC research were pain, site reaction, fatigue, nausea, and a single case of transient low blood pressure. Events resolved within three days on average. No other event was reported. These findings along with participants’ willingness to donate a second time supports that thesis that small volume bone marrow procurement is a safe and tolerable procedure.
This work was completed in compliance with Good Clinical Practice in Research standards set by the International Conference of Harmonization and under the Declaration of Helsinki.
- Gratwohl A, Niederwieser D. History of hematopoietic stem cell transplantation: evolution and perspectives. Curr Probl Dermatol. 2012; 43: 81-90. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22377922
- Zakirova EY, Valeeva AN, Aimaletdinov AM, Nefedovskaya LV, Akhmetshin RF, et al. Potential therapeutic application of mesenchymal stem cells in ophthalmology. Exp Eye Res. 2019; 189: 107863. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31669045
- Patel DM, Shah J, Srivastava AS. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int. 2013; 2013: 496218. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23577036
- Kassem M, Kristiansen M, Abdallah BM. Mesenchymal stem cells: cell biology and potential use in therapy. Basic Clin Pharmacol Toxicol. 2004; 95: 209-214. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15546474
- Gandini A, Roata C, Franchini M, Agostini E, Guizzardi E, et al. Unrelated allogeneic bone marrow donation: short- and long-term follow-up of 103 consecutive volunteer donors. Bone Marrow Transplant. 2001; 28: 369-374. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11571509
- Buckner CD, Clift RA, Sanders JE, Stewart P, Bensinger WI, et al. Marrow harvesting from normal donors. Blood. 1984; 64: 630-634. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/6380620
- Piccin A. Do we need to test blood donors for sickle cell anaemia? Blood Transfus. 2010; 8: 137-138. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20671871
- Chen SH, Wang TF, Yang KL. Hematopoietic stem cell donation. Int J Hematol. 2013; 97: 446-455. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23420184
- Pulsipher MA, Chitphakdithai P, Logan BR, Shaw BE, Wingard JR, et al. Acute toxicities of unrelated bone marrow versus peripheral blood stem cell donation: results of a prospective trial from the National Marrow Donor Program. Blood. 2013; 121: 197-206. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23109243
- Sakamaki H, Takamoto S, Shibata H. Complications of marrow harvesting for transplantation. Rinsho Ketsueki. 1994; 35: 29-35.
- Horowitz MM, Confer DL. Evaluation of Hematopoietic Stem Cell Donors. Hematology Am Soc Hematol Educ Program. 2005; 2005: 469-475. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16304422
- Bosi A, Bartolozzi B. Safety of bone marrow stem cell donation: a review. Transplant Proc. 2010; 42: 2192-2194. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20692441
- Miller JP, Perry EH, Price TH, Bolan CD Jr, Karanes C, et al. Recovery and safety profiles of marrow and PBSC donors: experience of the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008; 14: 29-36. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18721778
- Lisenko K, Stadtherr P, Bruckner T, Pavel P, Heilig CE, et al. Bone Marrow Harvesting of Allogeneic Donors in an Outpatient Setting: A Single-Center Experience. Biol Blood Marrow Transplant. 2016; 22: 470-474. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26551634
- Stroncek DF, Holland PV, Bartch G, Bixby T, Simmons RG, et al. Experiences of the first 493 unrelated marrow donors in the National Marrow Donor Program. Blood. 1993; 81: 1940-1946. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8461478
- Butterworth VA, Simmons RG, Bartsch G, Randall B, Schimmel M, et al. Psychosocial effects of unrelated bone marrow donation: experiences of the National Marrow Donor Program. Blood. 1993; 81: 1947-1959. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8461479
- Siddiq S, Pamphilon D, Brunskill S, Doree C, Hyde C, et al. Bone marrow harvest versus peripheral stem cell collection for haemopoietic stem cell donation in healthy donors. Cochrane Database Syst Rev. 2009; 1. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19160282
- Favre G, Beksac M, Bacigalupo A, Ruutu T, Nagler A, et al. Differences between graft product and donor side effects following bone marrow or stem cell donation. Bone Marrow Transplant. 2003; 32: 873-880. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14561987
- Nishimori M, Yamada Y, Hoshi K, Akiyama Y, Hoshi Y, et al. Health-related quality of life of unrelated bone marrow donors in Japan. Blood. 2002; 99: 1995-2001. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11877271
- Switzer GE, Bruce JG, Harrington D, Haagenson M, Drexler R, et al. Health-related quality of life of bone marrow versus peripheral blood stem cell donors: a prespecified subgroup analysis from a phase III RCT-BMTCTN protocol 0201. Biol Blood Marrow Transplant. 2014; 20: 118-127. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24184336
- Shaw BE, Logan BR, Kiefer DM, Chitphakdithai P, Pedersen TL, et al. An Analysis of the Effect of Race, Socioeconomic Status and Center Size on Unrelated NMDP Donor Outcomes: Donor Toxicities are More Common at Low Volume Bone Marrow Collection Centers. Biol Blood Marrow Transplant. 2015; 21: 1830-1838. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26116089
- Switzer GE, Dew MA, Butterworth VA, Simmons RG, Schimmel M. Understanding donors' motivations: a study of unrelated bone marrow donors. Soc Sci Med. 1997; 45: 137-147. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9203278
- Briggs NC, Piliavin JA, Lorentzen D, Becker GA. On willingness to be a bone marrow donor. Transfusion. 1986; 26: 324-330. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3523872
- Garcia MC, Chapman JR, Shaw PJ, Gottlieb DJ, Ralph A, et al. Motivations, Experiences, and Perspectives of Bone Marrow and Peripheral Blood Stem Cell Donors: Thematic Synthesis of Qualitative Studies. Biol Blood Marrow Transplant. 2013; 19: 1046-1058. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23603456
- Soule MC, Beale EE, Suarez L, Beach SR, Mastromauro CA, et al. Understanding Motivations to Participate in an Observational Research Study: Why do Patients Enroll? Soc Work Health Care. 2016; 55: 231-246. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26933943
- Squillaro T, Peluso G, Galderisi U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transplant. 2016; 25: 829-848. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26423725
- Sjostrom O, Holst D. Validity of a questionnaire survey: response patterns in different subgroups and the effect of social desirability. Acta Odontol Scand. 2002; 60: 136-140. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12166905
- Tishler CL, Bartholomae S. The Recruitment of Normal Healthy Volunteers: A Review of The Literature on the Use of Financial Incentives. J Clin Pharmacol. 2002; 42: 365-375. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11936560
- Grady C. Payment of clinical research subjects. J Clin Invest. 2005; 115: 1681-1687. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16007244
- Stunkel L, Grady C. More than the money: a review of the literature examining healthy volunteer motivations. Contemp Clin Trials. 2011; 32: 342-352. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21146635
- Boo M, van Walraven SM, Chapman J, Lindberg B, Schmidt AH, et al. Remuneration of hematopoietic stem cell donors: principles and perspective of the World Marrow Donor Association. Blood. 2011; 117: 21-25. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20921337
- Jean JE. Effects of accurate expectations about sensations on the sensory and distress components of pain. J Pers Soc Psychol. 1973; 27: 261-275. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/4723974
- Suls J, Wan CK. Effects of sensory and procedural information on coping with stressful medical procedures and pain: A meta-analysis. J Consult Clin Psychol. 1989; 57: 372-379. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2738210
- Pamphilon D, Siddiq S, Brunskill S, Dorée C, Hyde C, et al. Stem Cell Donation –What advice can be given to the donor? Br J Haematol. 2009; 147: 71-76.
- Kelley K, Clark B, Brown V, Sitzia J. Good practice in the conduct and reporting of survey research. Int J Qual Health Care. 2003; 15: 261-266. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12803354