About Onze Lieve Vrouwziekenhuis
Onze Lieve Vrouwziekenhuis
Articles by Onze Lieve Vrouwziekenhuis
European Clinical Laboratory, Molecular and Pathological (ECMP) criteria for prefibrotic JAK2V617F-Thrombocythemia and Polycythemia Vera versus MPL515- and CALR-Thrombocythemia and Myelofibrosis: From Dameshek to Michiels 1950-2018
Published on: 1st March, 2019
OCLC Number/Unique Identifier: 8056299472
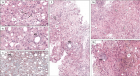
The broad spectrum of heterozygous versus homozygous JAK2V617F mutated MPN consists ET, ET with early features of PV (prodromal PV), classical PV, masked PV, advanced PV and post-PV myelofibrosis. Combined use of bone marrow histology and increased erythrocyte counts above 5.8x1012/L can replace increased red cell mass at time of presentation as the pathognomonic clue for the correct diagnosis of hetero/homozygous or homozygous mutated PV. Erythrocyte counts are in the normal range below 5.8x1012/L in heterozygous JAK2V617F mutated ET and prodromal PV but above 5.8x1012/L in heterozygous-homozygous or homozygous mutated PV. The bone marrow cellularity and morphology in pre-fibrotic ET, prodromal PV and PV carrying the JAK2V617F mutation are overlapping showing clustered increase of large mature pleomorphic megakaryocytes (M) with no increase of cellularity (<60%) in ET. The bone marrow is hypercellular (60%-80%) due to increased erythropoiesis megakaryopoiesis (EM) in prodromal and classical PV and trilinear hypercellular (80%-100% due increased megakaryopoiesis, erythropoiesis and granulopoiesis (EMG) in advanced PV and masked PV. Bone marrow cellularity ranging from normal (<60%) in ET to increased erythropoiesis (EM) in prodromal PV to hypercellular (80-100%) in advanced PV and masked PV largely depends on increasing JAK2V617F mutation load from low to high on top of other biological MPN variables like constitutional symptoms during long-term follow-up. MPL515 mutated ET is featured by an increase of clustered small and giant megakaryocytes with hyper-lobulated staghorn-like nuclei in a normal cellular bone marrow. The third entity of pronounced JAK2/MPL wild type ET associated with primary megakaryocytic granulocytic myeloproliferation (PMGM) without PV features proved to be caused by calreticulin (CALR) mutation. CALR mutated thrombocythemia is characterized by dual proliferation of megakaryocytic and granulocytic bone marrow proliferation of dense clustered large to giant immature dysmorphic megakaryocytes with bulky (bulbous) hyperchromatic nuclei, which are not seen in MPL515-mutated Thrombocythemia and JAK2V617F-Thrombocythemia, prodromal PV and classical PV.
Primary myelofibrosis is not primary anymore since the discovery of MPL515 and CALR mutations as driver causes of mono-linear megakaryocytic and dual megakaryocytic granulocytic myeloproliferation and secondary myelofibrosis
Published on: 15th April, 2019
OCLC Number/Unique Identifier: 8164054496
Primary myelofibrosis (PMF) is a distinct clinicopathological myeloproliferatve disease (MPD) not preceded by any other MPD ET, PV, CML,... Combined use of bone marrow histology and increased erythrocyte counts above 5.8x1012/L can replace increased red cell mass at time of presentation as the pathognomonic clue for the correct diagnosis of hetero/homozygous or homozygous mutated PV. Erythrocyte counts are in the normal range below 5.8x1012/L in heterozygous JAK2V617F mutated ET and prodromal PV but above 5.8x1012/L in heterozygous-homozygous or homozygous mutated PV. The bone marrow cellularity and morphology in pre-fibrotic ET, prodromal PV and PV carrying the JAK2V617F mutation are overlapping showing clustered increase of large mature pleomorphic megakaryocytes (M) with no increase of cellularity (<60%) in ET. The bone marrow is hypercellular (60%-80%) due to increased erythropoiesis megakaryopoiesis (EM) in prodromal and classical PV and trilinear hypercellular (80%-100% due increased megakaryopoiesis, erythropoiesis and granulopoiesis (EMG) in advanced PV and masked PV. Bone marrow cellularity ranging from normal (<60%) in ET to increased erythropoiesis (EM) in prodromal PV to hypercellular (80-100%) in advanced PV and masked PV largely depends on increasing JAK2V617F mutation load from low to high on top of other biological MPN variables like constitutional symptoms during long-term follow-up. MPL515 mutated ET is featured by an increase of clustered small and giant megakaryocytes with hyper-lobulated staghorn-like nuclei in a normal cellular bone marrow. The third entity of pronounced JAK2/MPL wild type ET associated with primary megakaryocytic granulocytic myeloproliferation (PMGM) without PV features proved to be caused by calreticulin (CALR) mutation. CALR mutated thrombocythemia is characterized by dual proliferation of megakaryocytic and granulocytic bone marrow proliferation of dense clustered large to giant immature dysmorphic megakaryocytes with bulky (bulbous) hyperchromatic nuclei, which are not seen in MPL515-mutated Thrombocythemia and JAK2V617F-Thrombocythemia, prodromal PV and classical PV.
The PVSG/WHO versus the Rotterdam European clinical, molecular and pathological diagnostic criteria for the classification of myeloproliferative disorders and myeloproliferative neoplasms (MPD/MPN): From Dameshek to Georgii, Vainchenker and Michiels 1950-2018
Published on: 17th April, 2019
OCLC Number/Unique Identifier: 8164019922
The present article extends the PVSG-WHO criteria into a simplified set of Rotterdam and European Clinical, Molecular and Pathological (RCP/ECMP) criteria to diagnose and classify the myeloproliferative neoplasms (MPNs). The crude WHO criteria still miss the masked and early stages of ET and PV. Bone marrow histology has a near to 100% sensitivity and specificity to distinguish thrombocythemia in BCR/ABL positive CML and ET, and the myelodysplastic syndromes in RARS-T and 5q-minus syndrome from BCR/ABL negative thrombocythemias in myeloproliferative disorders (MPD). The presence of JAK2V617F mutation with increased erythrocytes above 6x1012/L and hematocrit (>0.51 males and >0.48 females) is diagnostic for PV obviating the need of red cell mass measurement. About half of WHO defined ET and PMF and 95% of PV patients are JAK2V617F positive. The combination of molecular marker screening JAK2V617F, JAK2 exon 12, MPL515 and CALR mutations and bone marrow pathology is 100% sensitive and specific for the diagnosis of latent, early and classical ECMP defined MPNs. The translation of WHO defined ET, PV and PMF into ECMP criteria have include the platelet count above 350 x109/l, mutation screening and bone marrow histology as inclusion criteria for thrombocythemia in various MPNs. According to ECMP criteria, ET comprises three distinct phenotypes of true ET, ET with features of early (“forme fruste” PV), and ET with a hypercellular erythrocythemic, megakaryocytic granulocytic myeloproliferation (EMGM or masked PV). The ECMP criteria clearly differentiate early erythrocythemic, prodromal and classical PV from congenital polycythemia and idiopathic or secondary erythrocytosis. The burden of JAK2V617F mutation in heterozygous ET and in homozygous PV is of major clinical and prognostic significance. JAK2 wild type MPL515 mutated normocellular ET and MF lack PV features in blood and bone marrow. JAK2/MPL wild type hypercellular ET associated with primary megakaryocytic granulocytic myeloproliferation (PMGM) is the third distinct CALR mutated MPN. The translation of WHO into ECMP criteria for the classification of MPNs have a major impact on prognosis assessment and best choice for first line non-leukemogenic approach to postpone potential leukemogenic myelopsuppressive agents as long as possible in ET, PV and PMGM patients.
Bone marrow histology in CALR mutated thrombocythemia and myelofibrosis: Results from two cross sectional studies in 70 newly diagnosed JAK2/MPL wild type thrombocythemia patients
Published on: 21st June, 2019
OCLC Number/Unique Identifier: 8180078896
The clinical phenotypes in 268 JAK2V617F mutated MPN patients in the Seoul study were PV in 101, ET in 95 and MF in 78 and 56 CALR mutated MPN consisted of PV in none, ET in 40 and MF in 16 cases. CALR mutated MPN patients were younger than JAK2V617F mutated MPN patients (mean ages 57.5 and 66 years), had lower values for values for leukocytes (8.6 vs 11.9x109/L) and higher values for platelets (898 vs 643x109/L respectively). Bone marrow histopathology in 268 JAK2V617F mutated MPN patients in the Seoul study was featured by an increased erythropoiesis and megakaryopoiesis (EM) in 13.5%, an increased erythropoiesis, megakaryopoiesis and granulopoiesis (EMG) in 31.3%, a normocellular megakaryocytic (M) proliferation in 29,1%, a megakaryocytic and granulocytic (MG) proliferation with a relative reduction of erythropoiesis in post-ET and Post-PV myelofibrosis in 26.2%. The bone marrow histology in 56 cases of CALR mutated MPN show a predominantly increased megakaryopoiesis (M) in two thirds and an increased megakaryopoiesis and granulopoiesis (MG) with a decreased erythropoiesis in one third.
Thirteen consecutive CALR MPN patients in the Belgian & Dutch cross sectional study presented with thrombocythemia associated with a typical PMGM bone marrow histology in 11 and myelofibrosis in 2 cases. All 11 thrombocythemia and 2 myelofibrosis CALR mutated MPN patients did not have constitutional symptoms and did not suffer from microvascular erythromelalgic disturbances, major thrombosis at platelet counts between 400 and 1000x109/L. There was an occurrence of hemorrhages at platelet counts above 1000x109/L in two CALR thrombocythemia cases.
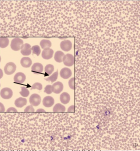
Bone marrow histology of CALR mutated thrombocythemia in the Seoul and Belgian/Dutch study showed loose clusters of large megakaryocytes (M) with bulky, cloud-like nuclei with a normal or a minor reduction of erythropoiesis and no increase in reticulin fibers grade 0 or 1 (RF 0 or 1). CALR thrombocythemia patients show various degrees of increased bone marrow cellularity due to dual megakaryocytic and granulocytic (MG) proliferation featured by large megakaryocytes with roundish bulky nuclear forms and cloud-like clumsy nuclei, which are almost never seen in JAK2V617F ET and PV. Assessment of allele burden is an independent and most important factor for all molecular variants MPN disease burden. Overt myelofibrosis with advanced post PV and or ET myelofibrosis at the bone marrow level occurred in one third (30%) of 208 evaluable JAK2 MPN patients and in 8 (14%) of 56 CALR MPN patients in the Seoul study.
Novel European Asiatic Clinical, Laboratory, Molecular and Pathobiological (2015-2020 CLMP) criteria for JAK2V617F trilinear polycythemia vera (PV), JAK2exon12 PV and JAK2V617F, CALR and MPL515 thrombocythemias: From Dameshek to Constantinescu-Vainchenker, Kralovics and Michiels
Published on: 3rd April, 2020
OCLC Number/Unique Identifier: 8576367174
The Myeloproliferative Neoplasms (MPN) of trilinear polycythemia vera (PV) and megakaryocytic leukemia (ML = primary megakaryocytic granulocytic myeloproliferation: PMGM) and Essential Thrombocythemia (ET) in the studies of Dameshek and Michiels are caused by the MPN driver mutations JAK2V617F, JAK2exon12, CALR and MPL515 discovered by Constantinescu-Vainchenker, Green and Kralovics. The JAK2V617F mutated trilinear myeloproliferative neoplasms (MPN) include a broad spectrum of clinical laboratory and bone marrow features in essential thrombocythemia (ET), prodromal PV and erythrocythemic PV, classical PV and advanced stages of masked PV and PV complicated by splenomegaly and secondary myelofibrosis (MF). Heterozygous JAK2V617F mutated ET is associated with low JAK2 allele and MPN disease burden and normal life expectance. In combined heterozygous and homozygous or homozygous JAK2V617F mutated trilinear PV, the JAK2 mutation load increases from less than 50% in prodromal PV and classical PV to above 50% up to 100% in hypercellular PV, advanced PV and PV with MF. Bone marrow histology show diagnostic features of eryhrocytic, megakaryocytic and granulocytic (EMG) myeloproliferation in JAK2V617F mutated trilinear MPN, which clearly differs from monolinear megakaryocytic (M) myelproliferation in MPL and CALR thrombocythemia and dual megakaryocytic granulocytic (MG) myeloproliferation in CALR mutated thrombocythemia. The morphology of clustered large pleomorphic megakaryocytes with hyperlobulated nuclei are similar in JAK2V617F thrombocythemia, prodromal PV and classical PV patients. Monolinear megakaryocytic (M) myeloproliferation of large to giant megakaryocytes with hyperlobulated staghorn-like nuclei is the hallmark of MPL515 mutated normocellular thrombocythemia. CALR mutated thrombocythemia usually presents with high platelet count around 1000x109/l and normocellular megakaryocytic (M) proliferation of immature megakaryocytes with cloud-like hyperchromatic nuclei followed by dual megakaryocytic granulocytic (MG) myeloproliferation followed by various degrees of bone marrow fibrosis. Natural history and life expectancy of MPN patients are related to the response to treatment and the degree of anemia, splenomegaly, myelofibrosis and constitutional symptoms. The acquisition of epigenetic mutations at increasing age on top of MPN disease burden independently predict unfavorable outcome in JAK2V617F, MPL515 and CALR mutated myeloproliferative neoplasms (MPNs, which mutually exclude each other).

If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."