Abstract
Research Article
European Clinical Laboratory, Molecular and Pathological (ECMP) criteria for prefibrotic JAK2V617F-Thrombocythemia and Polycythemia Vera versus MPL515- and CALR-Thrombocythemia and Myelofibrosis: From Dameshek to Michiels 1950-2018
Jan Jacques Michiels*, Zwi Berneman, Wilfried Schroyens, Fibo W J ten Kate, King Lam and Hendrik De Raeve
Published: 01 March, 2019 | Volume 2 - Issue 1 | Pages: 001-017
The broad spectrum of heterozygous versus homozygous JAK2V617F mutated MPN consists ET, ET with early features of PV (prodromal PV), classical PV, masked PV, advanced PV and post-PV myelofibrosis. Combined use of bone marrow histology and increased erythrocyte counts above 5.8x1012/L can replace increased red cell mass at time of presentation as the pathognomonic clue for the correct diagnosis of hetero/homozygous or homozygous mutated PV. Erythrocyte counts are in the normal range below 5.8x1012/L in heterozygous JAK2V617F mutated ET and prodromal PV but above 5.8x1012/L in heterozygous-homozygous or homozygous mutated PV. The bone marrow cellularity and morphology in pre-fibrotic ET, prodromal PV and PV carrying the JAK2V617F mutation are overlapping showing clustered increase of large mature pleomorphic megakaryocytes (M) with no increase of cellularity (<60%) in ET. The bone marrow is hypercellular (60%-80%) due to increased erythropoiesis megakaryopoiesis (EM) in prodromal and classical PV and trilinear hypercellular (80%-100% due increased megakaryopoiesis, erythropoiesis and granulopoiesis (EMG) in advanced PV and masked PV. Bone marrow cellularity ranging from normal (<60%) in ET to increased erythropoiesis (EM) in prodromal PV to hypercellular (80-100%) in advanced PV and masked PV largely depends on increasing JAK2V617F mutation load from low to high on top of other biological MPN variables like constitutional symptoms during long-term follow-up. MPL515 mutated ET is featured by an increase of clustered small and giant megakaryocytes with hyper-lobulated staghorn-like nuclei in a normal cellular bone marrow. The third entity of pronounced JAK2/MPL wild type ET associated with primary megakaryocytic granulocytic myeloproliferation (PMGM) without PV features proved to be caused by calreticulin (CALR) mutation. CALR mutated thrombocythemia is characterized by dual proliferation of megakaryocytic and granulocytic bone marrow proliferation of dense clustered large to giant immature dysmorphic megakaryocytes with bulky (bulbous) hyperchromatic nuclei, which are not seen in MPL515-mutated Thrombocythemia and JAK2V617F-Thrombocythemia, prodromal PV and classical PV.
Read Full Article HTML DOI: 10.29328/journal.ijbmr.1001002 Cite this Article Read Full Article PDF
References
- Michiels JJ. Physiopathology, etiologic factors, diagnosis and course of polycythemia vera as related to therapy according to William Dameshek 1940-1950. Turkish J Hematol. 2013; 30: 102-110. Ref.: https://goo.gl/VWF3Td
- Michiels JJ, Ten Kate FWJ, Vuzevski VD, Abels J. Histopathology of erythromelalgia in thrombocythemia. Histopathology. 1984; 8: 669-678. Ref.: https://goo.gl/t79oZi
- Michiels JJ, Abels J, Steketee J, van Vliet HHDM, Vuzevski VD. Erythromelalgia caused by platelet mediated arteriolar inflammation and thrombosis in thrombocythemia. Ann Intern Med. 1985; 102: 466-471. Ref.: https://goo.gl/SctHNS
- Michiels JJ, Koudstaal PJJ, Mulder AH, Van Vliet HHDM. Transient neurologic and ocular manifestations in primary thrombocythemia. Neurology. 1993; 43: 1107-1110. Ref.: https://goo.gl/cYQy8p
- Michiels JJ, Juvonen E. Proposal for revised diagnostic criteria of essential thrombocythemia and polycythemia vera by the Thrombocythemia Vera Study Group (TVSG). Semin Thromb Hemostas. 1997; 23: 339-347. Ref.: https://goo.gl/9igXoi
- Michiels JJ, Thiele J. Clinical and pathological criteria for the diagnosis of essential thrombocthemia, polycythemia vera, and idiopathic myelofibrosis (agnogenic myeloid metaplasia). Int J Hematol. 2002; 76: 133-145. Ref.: https://goo.gl/nZkVe3
- Michiels JJ, De Raeve H, Hebeda K, Lam KH, Berneman Z, et al. WHO bone marrow features and European clinical molecular and pathlogical (EMCP) criteria for the diagnosis and classification of myeloproliferative disorders. Leuk Res. 2007; 31: 1031-1038. Ref.: https://goo.gl/xNUgyu
- Laszlo J. Myeloproliferative disorders (MPD): myelofibrosis, myelosclerosis, extramedullary hematopoiesis, undifferentiated MPD, and primary hemorrhagic thrombocythemia. Semin Haematol. 1975; 12: 409-432. Ref.: https://goo.gl/8pu88V
- Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951; 6: 372-375. Ref.: https://goo.gl/VVzyAJ
- Berlin MI. Diagnosis and classification of the polycythemias. Semin Hematol. 1975; 12: 339-351. Ref.: https://goo.gl/U5Yaad
- Ellis JT, Silver RT, Coleman M, Geller SA. The bone marrow in polycythemia vera. Semin Hematol. 1986; 12: 433-444. Ref.: https://goo.gl/fNNG28
- Kurnick JE, Ward HP, Block MH. Bone marrow sections in the differential diagnosis of polycythemia. Arch Pathol. 1972; 94: 489-499. Ref.: https://goo.gl/8vjxEJ
- Michiels JJ, Kutti J, Stark P, Bazzan M, Gugliotta L, et al. Diagnosis, pathogenesis and treatment of the myeloproliferative disorders essential thromboythemia, polycythemia vera and essential megakaryocytic granulocytic myeloproliferation and myelofibrosis. Neth J Med. 1999; 54: 46-62. Ref.: https://goo.gl/VbWuP2
- Michiels JJ, Barbui T, Finazzi G, Fruchtman SM, Kutti J, et al. Diagnosis and treatment of polycythemia vera and possible future study designs of the PVSG. Leukemia Lymphoma. 2000; 36: 239-253. Ref.: https://goo.gl/JA8arG
- Michiels JJ. Bone marrow histopathology and biological markers as specific clues to differential diagnosis of essential thrombocythemia, polycythemia vera and prefibrotic or fibrotic agnogenic myeloid metaplasia. The Hematol J. 2004; 5: 93-102. Ref.: https://goo.gl/C7nKXe
- Michiels JJ, Kvasnicka HM, Thiele J. Myeloproliferative Disorders. Current perspectives on diagnostic criteria, histopathology and treatment in essential thrombocythemia, polycythemia vera, and chronic idiopathic myelofibrosis. ISBN 3-9808075-6-8, 2005. Ref.: https://goo.gl/UvMStz
- Thiele J, Kvasnicka HM, Facchetti F, Franco V, Van Der Walt J, et al. European consensus for grading bone marrow fibrosis and assessment of cellularity in myeloproliferative diaorders. Haematologica. 2005; 90: 1128-1132. Ref.: https://goo.gl/AEWjiW
- Baxter EJ1, Scott LM, Campbell PJ, East C, Fourouclas N, et al. Acquired mutation of tyrosine kinase in human myeloproliferative disorders. Lancet. 2005; 365: 1054-1061. Ref.: https://goo.gl/JRaA1r
- Messinezy M, Westwood NB, Woodcock SP, Strong RM, Pearson TC. Low serum erythropoietin - a strong diagnostic criterion of primary polycythaemia even at normal haemoglobin levels. Clin Lab Haematol. 1995; 17: 217-220. Ref.: https://goo.gl/4QfJiu
- Messinezy M1, Westwood NB, El-Hemaidi I, Marsden JT, Sherwood RS, et al. Serum erythropoietine values in erythrocytoses and in primary thrombocythemia. Br J Haematol. 2002; 117: 47-53. Ref.: https://goo.gl/b3vQaJ
- Mossuz P1, Girodon F, Donnard M, Latger-Cannard V, Dobo I, et al. Diagnostic value of serum erythropoietine in patients with absolute erythrocytosis. Haematologica. 2004; 89: 1194-1198. Ref.: https://goo.gl/ezvfQq
- Dameshek W, Henstell HH. The diagnosis of polycythemia. Ann Intern Med. 1940: 13: 1360-1387. Ref.: https://goo.gl/9UY1MD
- Dameshek W. Physiopathology and course of polycythemia vera as related to therapy. JAMA. 1950; 142: 790-797. Ref.: https://goo.gl/sNRiJJ
- Thiele et al. 2008 WHO criteria for polycthemia vera, primary myelofibrosis and essential thrombocythemia. In: Swerdlow SH, Campo E, Harris NL, et al: WHO Classification of Tumours of Haematopoietic and Lympoid Tissues. Lyon France IARC Press. 2008; 40-50.
- Thiele J, Zankovich R, Schneider G, Kremer B, Fischer R, et al. Primary (essential) thrombocythemia versus polycythemia rubra vera. A histmorphometric analysis of bone marrow features in trephine biopsies. Anal Quant Cytol Histol. 1988; 10: 375-382. Ref.: https://goo.gl/MLursT
- Georgii A, Vykoupil KF, Buhr H, Choritz H, Doehler U, et al. Chronic myeloproliferative disorders in bone marrow biopsies. Path Res Pracxt. 1990; 186: 3-27. Ref.: https://goo.gl/mU4E5g
- Piche A, Riera L, Beggiato E, Nicolino B, Godio L, et al. JAK2V617F mutation and allele burden are associated with distinct clinical and morphological subtypes in patients with essential thrombocythemia. J Clin Pathol. 2012; 65: 953-954. Ref.: https://goo.gl/UrE18X
- Thiele, Kvasnicka HM. Chronic myeloproliferative disorders with thrombocythemia: a comparative study of two classification systems (PVSG, WHO) on 839 patients. Ann Hematol. 2003; 82: 148-152. Ref.: https://goo.gl/tv1drc
- Tefferi A1, Thiele J, Orazi A, Kvasnicka HM, Barbui T, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood. 2007; 110: 1092-1097. Ref.: https://goo.gl/HRpXUq
- Buhr T, Hebeda K, Kaloutsi V, Porwit A, Van der Walt J, et al. European Bone Marrow Working group trial to discriminate essential thrombocythemia from prefibrotic primary myelofibrosis. Haematologica. 2012; 97: 360-365. Ref.: https://goo.gl/3tXFk5
- Michiels JJ, Commandeur S, Hoogenboom GJ, Wegman JJ, Scholten L, et al. JAK2V617F positive early stage myeloproliferative disease (essential thrombocythemia) as the cause of portal vein thrombosis in two middle-aged women: therapeutic implications in view of the literature. Ann Hematol. 2007; 86: 793-800. Ref.: https://goo.gl/CCwbSU
- James C1, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, et al including Constantinescu S, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signaling causes polycythemia var. Nature. 2005; 434: 1144-1148. Ref.: https://goo.gl/qHQJYj
- Vainchenker W, Constantinescu SN. A unique activating mutation in JAK2 (V617F) is at the origin of polycythemia vera and allows a new classification of myeloproliferative diseases. Hematology (Am Soc Hematol Educ Progr). 2005; 195-200. Ref.: https://goo.gl/5vUrwS
- Michiels JJ, De Raeve H, Berneman Z, Van Bockstaele D, Hebeda K, et al. The 2001 WHO and updated European Clinical and Pathological (ECP) criteria for the diagnosis, classification, and staging of the Philadelphia chromosome-negative chronic myeloproliferative disorders (CMPD). Sem Thromb Hemostas. 2006; 32: 307-340. Ref.: https://goo.gl/XNy2Qe
- Michiels JJ, Berneman Z, Van Bockstaele, Van Der Planken M, De Raeve H, et al. Clinical and laboratory features, pathobiology of platelet-mediated thrombosis and bleeding complications, and the molecular etiology of essential thrombocythemia and polycythemia vera: therapeutic implications. Semin Thromb Hemostas. 2006; 32: 174-207. Ref.: https://goo.gl/8WHXgk
- Villeval JL, James C, Pisani D, Casadevall N, Vainchenker W. New insights into pathogenesis of JAK2V617F-positive myeloproliferative disorders and consequences for the management of patients. Sem Thromb Hemostas. 2006; 32: 341-351. Ref.: https://goo.gl/KzCMz2
- Thiele J, Kvasnicka HM, Diehl V. Initial (latent) polycythemia vera with thrombocytosis mimicking essential thrombocythemia. Acta Haematol. 2005; 113: 213-219. Ref.: https://goo.gl/ZjSa5L
- Gale RE, Allen AJR, Nash MJ, Linch DC. Log-term serial analysis of X-chromosome inactivation patterns andJAK2 V617F mutant levels in patients with essential thrombocythemia show that minor mutant-positive clones can remain stable for many years. Blood. 2007; 109: 1241-1243. Ref.: https://goo.gl/wBiyPZ
- Campbell PJ1, Scott LM, Buck G, Wheatley K, East CL, et al. Definition of essential thrombocythemia and relation of essential thrombocythemia to polycythemia vera based on JAK2 V617F mutation: a prospective study. Lancet. 2005; 366: 1945-1953. Ref.: https://goo.gl/nfWb6b
- Vannucchi AM1, Antonioli E, Guglielmelli P, Rambaldi A, Barosi G, et al. Clinical profile of homozygous JAK2V617F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007; 110: 840-846. Ref.: https://goo.gl/SC6fAj
- Antonioli E1, Guglielmelli P, Poli G, Bogani C, Pancrazzi A,, et al. Influence of JAK2V617F allele burden on phenotype in essential thrombocythemia. Haematologica. 2008; 93: 41-48. Ref.: https://goo.gl/5EXWA5
- Vannucchi AM1, Antonioli E, Guglielmelli P, Pancrazzi A, Guerini V, et al. Charateristics and clinical correlates of MPL515W>L/K mutation in essential thrombocythemia. Blood. 2008; 112: 844-847. Ref.: https://goo.gl/2bmSWK
- Beer PA1, Campbell PJ, Scott LM, Bench AJ, Erber WN, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood. 2008; 112: 141-149. Ref.: https://goo.gl/hVpnbo
- Klampf T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, et al. Somatic mutations od calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013; 369: 2379-2390. Ref.: https://goo.gl/eWxPi3
- Georgii A, Buhr H, Buesche G, Kreft A, Chorotz H. Classification and staging of Ph-negative chronic myeloproliferative diseases. Leuk Lymphoma. 1996; 22(suppl 1): 15-29. Ref.: https://goo.gl/vBXsjq
- Georgii A, Buesche G, Kreft A. The histopathology of chronic myeloproliferative diseases. Bailliere’s Clin Haematol. 1998; 11: 721-749. Ref.: https://goo.gl/a5Fzas
- Michiels JJ, Valster F, Wielenga J, Schelfout K, De Raeve H. European vs 2015 World Health Organization clinical molecular and pathological (WHO-CMP) classification of myeloproliferative neoplasms. World J Hematol. 2015; 4: 16-53. Ref.: https://goo.gl/iaCLC6
- Michiels JJ, Berneman Z, Schroyens W, De Raeve H. Changing concepts of diagnostic criteria of myeloproliferative disorders and the molecular etiology and classification of myeloproliferative neoplasms: From Dameshek 1950 to Vainchenker 2005 and beyond. Acta Haematol. 2015; 133: 36-51. Ref.: https://goo.gl/CJRMmt
- Michiels JJ, Tevet M, Trifa A, Niculescu-Mizil E, Lupa A, et al. 2016 WHO Clinical Molecular and Pathological Criteria for Classification and Staging of Myeloproliferative Neoplasms (MPN) Caused by MPN Driver Mutations in the JAK2, MPL and CALR Genes in the Context of New 2016 WHO Classification: Prognostic and Therapeutic Implications. MAEDICA. 2016; 11: 5-25. Ref.: https://goo.gl/wJH2oJ
- Michiels JJ, De Rave H, Valster F, Potters V, Kim Y, et al. Extension of 2016 World Health Organization (WHO) classification and a new set of clinical, laboratory, molecular and pathological (CLMP) criteria for the diagnosis of myeloproliferative neoplasms: from Dameshek to Vainchenker, Green and Kralovics. EMJ. 2017a; 2: 72-81. Ref.: https://goo.gl/3QED5Z
- De Raeve H, Michiels JJ, Valster F, Potters V, Kim Y, et al. Novel clinical, laboratory, mollecular and pathological (2018 CLMP) criteria for the differential diagnosis of three distinct myeloprproliferative neoplasms: the role of driver mutation analysis and bone marrow histology. Int J Cancer Res Ther. 2018; 3: 1-13.
- De Raeve H, Fostier K, Valster F, Potters V, Kim Y, et al. Bone marrow histology is a pathognomonic clue to each of the JAK2V617F, MPL515 and CALR mutated thrombocythemia in myeloproliferative neoplasms. Clin Res Hematol. 2018; 1: 1-7. Ref.: https://goo.gl/hq1ZCN
Figures:
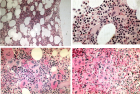
Figure 1

Figure 2

Figure 3
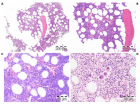
Figure 4
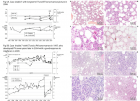
Figure 5
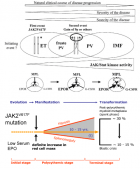
Figure 6
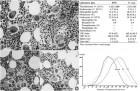
Figure 7
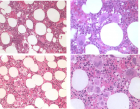
Figure 8
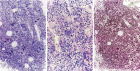
Figure 9
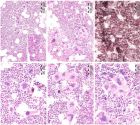
Figure 10

Figure 11
Similar Articles
-
Pure Erythroid Leukemia: The Sole Acute Erythroid LeukemiaFauzia Shafi Khan*,Khalid Mahmood,Alia Ahmad. Pure Erythroid Leukemia: The Sole Acute Erythroid Leukemia. . 2017 doi: 10.29328/journal.ijbmr.1001001; 1: 001-005
-
European Clinical Laboratory, Molecular and Pathological (ECMP) criteria for prefibrotic JAK2V617F-Thrombocythemia and Polycythemia Vera versus MPL515- and CALR-Thrombocythemia and Myelofibrosis: From Dameshek to Michiels 1950-2018Jan Jacques Michiels*,Zwi Berneman,Wilfried Schroyens,Fibo W J ten Kate,King Lam,Hendrik De Raeve. European Clinical Laboratory, Molecular and Pathological (ECMP) criteria for prefibrotic JAK2V617F-Thrombocythemia and Polycythemia Vera versus MPL515- and CALR-Thrombocythemia and Myelofibrosis: From Dameshek to Michiels 1950-2018. . 2019 doi: 10.29328/journal.ijbmr.1001002; 2: 001-017
-
Primary myelofibrosis is not primary anymore since the discovery of MPL515 and CALR mutations as driver causes of mono-linear megakaryocytic and dual megakaryocytic granulocytic myeloproliferation and secondary myelofibrosisJan Jacques Michiels*,Hendrik De Raeve. Primary myelofibrosis is not primary anymore since the discovery of MPL515 and CALR mutations as driver causes of mono-linear megakaryocytic and dual megakaryocytic granulocytic myeloproliferation and secondary myelofibrosis. . 2019 doi: 10.29328/journal.ijbmr.1001003; 2: 018-026
-
The PVSG/WHO versus the Rotterdam European clinical, molecular and pathological diagnostic criteria for the classification of myeloproliferative disorders and myeloproliferative neoplasms (MPD/MPN): From Dameshek to Georgii, Vainchenker and Michiels 1950-2018Jan Jacques Michiels*,Hendrik De Raeve. The PVSG/WHO versus the Rotterdam European clinical, molecular and pathological diagnostic criteria for the classification of myeloproliferative disorders and myeloproliferative neoplasms (MPD/MPN): From Dameshek to Georgii, Vainchenker and Michiels 1950-2018. . 2019 doi: 10.29328/journal.ijbmr.1001004; 2: 027-050
-
The forgotten player in the surgical historyYves Cirotteau*. The forgotten player in the surgical history. . 2019 doi: 10.29328/journal.ijbmr.1001005; 2: 051-063
-
Bone marrow histology in CALR mutated thrombocythemia and myelofibrosis: Results from two cross sectional studies in 70 newly diagnosed JAK2/MPL wild type thrombocythemia patientsJan Jacques Michiels*,Yonggoo Kim,Myungshin Kim,Francisca Valster,Vincent Potters,Zwi Berneman,Alain Gadisseur,Wilfried Schroyens,Hendrik De Raeve. Bone marrow histology in CALR mutated thrombocythemia and myelofibrosis: Results from two cross sectional studies in 70 newly diagnosed JAK2/MPL wild type thrombocythemia patients. . 2019 doi: 10.29328/journal.ijbmr.1001006; 2: 064-078
-
Serum MicroRNA-155 in Acute Graft-Versus-Host-Disease (aGVHD)Yvonne A Efebera*,Amy S Ruppert,Apollinaire Ngankeu,Sabrina Garman,Prasanthi Kumchala,Alan Howard,Steven M Devine,Parvathi Ranganathan,Ramiro Garzon. Serum MicroRNA-155 in Acute Graft-Versus-Host-Disease (aGVHD). . 2019 doi: 10.29328/journal.ijbmr.1001007; 2: 079-082
-
Drug abuse and its ramifications on skeletal systemDas Sanjita*,Kumar Naveen. Drug abuse and its ramifications on skeletal system. . 2019 doi: 10.29328/journal.ijbmr.1001008; 2: 083-086
-
Correlation of plasma protein from MDS, young and elderly patients by SDS-pageNathanielly de Lima Silva*,Josiel Nascimento dos Santos,Márcia Santos Rezende,Lúcio Henrique Sousa Pinheiro,Carlos Arthur Cardoso Almeida,Dulce Marta Schimieguel,Danilo Nobre. Correlation of plasma protein from MDS, young and elderly patients by SDS-page. . 2019 doi: 10.29328/journal.ijbmr.1001009; 2: 087-088
-
The motivational factors and adverse events experienced by healthy volunteers donating bone marrow for researchMirella Ejiugwo,Georgina Shaw,Frank Barry,Janusz Krawczyk,Veronica McInerney*. The motivational factors and adverse events experienced by healthy volunteers donating bone marrow for research. . 2019 doi: 10.29328/journal.ijbmr.1001010; 2: 089-096
Recently Viewed
-
Physical Performance in the Overweight/Obesity Children Evaluation and RehabilitationCristina Popescu, Mircea-Sebastian Șerbănescu, Gigi Calin*, Magdalena Rodica Trăistaru. Physical Performance in the Overweight/Obesity Children Evaluation and Rehabilitation. Ann Clin Endocrinol Metabol. 2024: doi: 10.29328/journal.acem.1001030; 8: 004-012
-
Hypercalcaemic Crisis Associated with Hyperthyroidism: A Rare and Challenging PresentationKarthik Baburaj*, Priya Thottiyil Nair, Abeed Hussain, Vimal MV. Hypercalcaemic Crisis Associated with Hyperthyroidism: A Rare and Challenging Presentation. Ann Clin Endocrinol Metabol. 2024: doi: 10.29328/journal.acem.1001029; 8: 001-003
-
Overlap of two unusual condition in childhood: hibernoma and central diabetes insipidusKübra ARSLAN*,Ayça TÖREL ERGÜR,Mehmet Ali YİNANÇ. Overlap of two unusual condition in childhood: hibernoma and central diabetes insipidus. Ann Clin Endocrinol Metabol. 2022: doi: 10.29328/journal.acem.1001023; 6: 001-003
-
Effective treatment of diabetes mellitus and autoimmune diseases by resonance medicinePraznikov Victor*. Effective treatment of diabetes mellitus and autoimmune diseases by resonance medicine. Ann Clin Endocrinol Metabol. 2022: doi: 10.29328/journal.acem.1001024; 6: 004-009
-
Thyroid NodulesRajiv Datta*. Thyroid Nodules. Ann Clin Endocrinol Metabol. 2022: doi: 10.29328/journal.acem.1001025; 6: 010-010
Most Viewed
-
Impact of Latex Sensitization on Asthma and Rhinitis Progression: A Study at Abidjan-Cocody University Hospital - Côte d’Ivoire (Progression of Asthma and Rhinitis related to Latex Sensitization)Dasse Sery Romuald*, KL Siransy, N Koffi, RO Yeboah, EK Nguessan, HA Adou, VP Goran-Kouacou, AU Assi, JY Seri, S Moussa, D Oura, CL Memel, H Koya, E Atoukoula. Impact of Latex Sensitization on Asthma and Rhinitis Progression: A Study at Abidjan-Cocody University Hospital - Côte d’Ivoire (Progression of Asthma and Rhinitis related to Latex Sensitization). Arch Asthma Allergy Immunol. 2024 doi: 10.29328/journal.aaai.1001035; 8: 007-012
-
Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian RandomizationYong-Qing Zhu, Xiao-Yan Meng, Jing-Hua Yang*. Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian Randomization. Arch Asthma Allergy Immunol. 2023 doi: 10.29328/journal.aaai.1001032; 7: 012-022
-
An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergiesNathalie Cottel,Aïcha Dieme,Véronique Orcel,Yannick Chantran,Mélisande Bourgoin-Heck,Jocelyne Just. An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergies. Arch Asthma Allergy Immunol. 2021 doi: 10.29328/journal.aaai.1001027; 5: 030-037
-
Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trialSathit Niramitmahapanya*,Preeyapat Chattieng,Tiersidh Nasomphan,Korbtham Sathirakul. Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trial. Ann Clin Endocrinol Metabol. 2023 doi: 10.29328/journal.acem.1001026; 7: 00-007
-
Physical Performance in the Overweight/Obesity Children Evaluation and RehabilitationCristina Popescu, Mircea-Sebastian Șerbănescu, Gigi Calin*, Magdalena Rodica Trăistaru. Physical Performance in the Overweight/Obesity Children Evaluation and Rehabilitation. Ann Clin Endocrinol Metabol. 2024 doi: 10.29328/journal.acem.1001030; 8: 004-012

If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."